 Producers of intragastric balloons, which are used in non-invasive weight loss procedures, are coming under investigation for a total of twelve deaths attributed to their use.
Producers of intragastric balloons, which are used in non-invasive weight loss procedures, are coming under investigation for a total of twelve deaths attributed to their use.
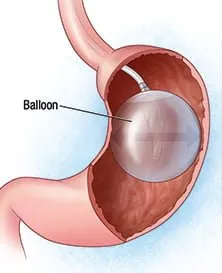
These balloons are placed in one’s stomach through an endoscopic procedure via the patient’s mouth. They are then filled with saline to take up space in the patient’s stomach, making them eat less, feel fuller, and helping them loss weight. The balloons are removed after six months, usually resulting in a 5%-10% loss of the patient’s total body weight.
The two products in question are the Orbera Intragastric Balloon, manufactured by Apollo Endosurgery Inc. and the Integrated Dual Balloon, which is manufactured by ReShape Lifesciences.
One of the twelve deaths can be linked to esophageal perforation. Four of the twelve suffered gastric perforation, but the seven others do not have a particular cause of death. That being said, there are several complications with intragastric balloons that may be the culprit. One of these is over-inflation. This is where the balloon is overfilled with saline during the surgery, and it causes the stomach to stretch, leak acid, or rupture as a result.
These other symptoms can be ascribed to over inflation of intragastric balloons. If you or a loved one has experienced any of the following, consult your physician immediately:
- Vomiting
- Difficulty breathing
- Stomach pain
- Tenderness
Severe abdominal and back pain have also been reported with the use of these balloons, although they have not been traced to any of the twelve deaths. Furthermore, these symptoms are not currently on the balloon’s warning label, which means that doctors have a harder time attributing the problem to it. Lastly, the balloon has been known to cause acute pancreatitis (inflammation of the pancreas) by “the compression of gastrointestinal structures created by the implanted balloon(s).” A diagnosis of pancreatitis would mean that the balloon was not implanted in the patient properly. This, too, has not been traced back specifically to any of the twelve deaths.
The FDA released warnings to health care providers about the potential risks of such intragastric balloons in February, 2016 and again in August, 2016. [READ THEM HERE AND HERE]






