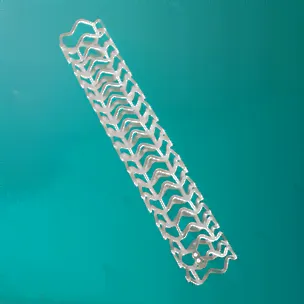
 The Food and Drug Administration (FDA) distributed a warning letter to physicians concerning the risks of using Abbott Vascular’s Absorb GT1 Bioresorbable Vascular Scaffold (BVS) on March 18th, 2017. The BVS is a stent placed into one’s coronary artery to widen it and increase blood flow. First, it is placed on a catheter over a balloon, which is inserted into one’s artery through their groin or arm. The balloon fills with air, which, in turn, widens the stent and, by extension, the artery to the desired diameter. The balloon and the catheter are removed, leaving the stent in place. It dissolves after three years. BVS is made of various types of polymers and is used often in coronary bypass surgeries.
The Food and Drug Administration (FDA) distributed a warning letter to physicians concerning the risks of using Abbott Vascular’s Absorb GT1 Bioresorbable Vascular Scaffold (BVS) on March 18th, 2017. The BVS is a stent placed into one’s coronary artery to widen it and increase blood flow. First, it is placed on a catheter over a balloon, which is inserted into one’s artery through their groin or arm. The balloon fills with air, which, in turn, widens the stent and, by extension, the artery to the desired diameter. The balloon and the catheter are removed, leaving the stent in place. It dissolves after three years. BVS is made of various types of polymers and is used often in coronary bypass surgeries.
Interim five-year clinical study results show that the BVS causes a disproportionate amount of major cardiac events (such as heart failure) and scaffold thrombosis–clotting in the artery–after merely three years. Clotting was found to be over three times higher in patients implanted with the BVS than with the metallic XIENCE drug-eluting stent, which has been approved wholeheartedly by the FDA, does not get absorbed into one’s body, and, coincidentally, is also manufactured by Abbott. XIENCE is implemented in much the same way as the BVS. 13.4% of patients treated with the BVS experienced a major cardiac event three years after their procedure, versus 10.4% of those treated with the XIENCE stent. Other studies show that the BVS fails more often due to suboptimal implantation [READ ABOUT IT HERE]. The clinical trials will persist for the total five years to show the long-term effects of BVS implantation.
Presumably as a result of the startling findings, Abbott has canceled all global sales of the BVS as of September 2017, citing “low commercial sales”. The FDA relayed this news to physicians through another, updated letter, and they recommends the following for doctors who still plan on using their leftover BVSs (These standards are unchanged from their March letter to physicians.):
- Follow the instructions for target heart vessel selection (e.g., avoiding BVS use in small heart vessels) and optimal device implantation that are included in the BVS physician labeling.
- Advise patients experiencing any new cardiac symptoms such as irregular heartbeats, chest pain, or shortness of breath to seek clinical care. For more information about risks associated with the BVS, refer to the BVS physician labeling.
- Advise BVS patients to follow the recommendations for dual antiplatelet therapy (DAPT) prescribed by their health care providers.
- Report any adverse events related to the BVS that come to your attention. If you suspect a problem with the BVS, we encourage you to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by facilities that are subject to the FDA’s user facility reporting requirements should follow the reporting procedures established by their facilities
Sources:
file:///Users/garrettdowell/Downloads/absorb-bioresorbable-scaffold-dissolving-stent%20(1).html






